Is contamination of endoscopes really a problem?
More nosocomial infection and pseudo-infection outbreaks have been linked to contaminated endoscopes than to any other medical device.1
The results of an investigation conducted by a U.S. Senate committee proves that between 2012 and the spring of 2015, endoscopes caused at least 250 life-threatening infections worldwide, including infections with the superbug carbapenem-resistant enterobacteriaceae.2
Contamination and inadequate cleaning of endoscopes has been recognized as a major patient safety issue by the ECRI - a non-profit US organization focusing on improving patient safety. Therefore, the annual ECRI list of "Top 10 Health Technology Hazards” has included this issue continuously for the past 9 years.3
Would you like more information?
Biofilm risk in reusable endoscopes
Routine cleaning procedures do not remove biofilm reliably from endoscope channels.21
Therefore, implementation of infection control through microbiological surveillance of endoscope reprocessing is appropriate to detect early colonization and biofilm formation in the endoscope and to prevent contamination and infection in patients after endoscopic procedures.22
FDA requires infection control
On March 12, 2015 the U.S. Food and Drug Administration announced new actions to enhance the safety of reusable medical devices and address the possible spread of infectious agents between usages.4, 5, 6
Safety communication on bronchoscopes by FDA
Since then the FDA has issued a new guidance on reprocessing of medical devices, hosted a 2-day seminar discussing the transmission of infection associated with endoscopes, issued warnings to duodenoscope manufacturers for lack of filing MDR reports, as well as issued a safety communication on the infection associated with reprocessed flexible bronchoscopes.6
FDA interventions
March, 2015:
New guidance on reprocessing of medical device
May, 2015:
2-day seminar to address problems with contaminated endoscopes
August, 2015:
Postmarket surveillance studies on endoscopes required
August, 2015:
Warnings to duodenoscope manufacturers
September, 2015:
Safety communication on bronchoscopes
November, 2016:
Two patients died due to a contaminated bronchoscope in a US facility7
January, 2017:
MDR on contaminated bronchoscopes breaks the record with 183 MDRs reported to FDA in 2016
January, 2018:
MDR on contaminated bronchoscopes breaks a new record with 215 MDRs reported to FDA in 2017
March, 2018:
FDA warns duodenoscope manufacturers about failure to perform postmarket surveillance studies to assess contamination risk
Continuous increase in non-compliance with an infection control standard intended to reduce infection risk
IC.02.02.01 Non-compliance of US hospitals
The Joint Commission accredits and certifies nearly 21,000 health care organizations and programs in the United States.
The Joint Commission Standard IC.02.02.01: requires organizations to reduce the risk of infections associated with medical equipment, devices and supplies. Since 2009, there has been a continuous increase in non-compliance with IC.02.02.01 as equipment is improperly high level disinfected (HLD) and sterilized.
"To this point, The Joint Commission has found that from 2013-2016, immediate threat to life (ITL) declarations directly related to improperly sterilized or HDL equipment increased significantly. In 2016, 74 percent of all ITLs were related to improperly sterilized or HDL equipment." 23
Infected bronchoscopes pose a particular challenge
Flexible bronchoscopes are difficult to clean and disinfect due to their long, narrow lumens and delicate materials. The question is whether it is possible to ensure 100% disinfection of each scope. Despite following cleaning instructions persistent device contamination has been seen, and failure to meticulously follow cleaning instructions is likely to lead to contaminated scopes.6, 8
Routine cleaning is not enough
The true incidence of cross-contamination and infection during flexible bronchoscopy is likely under-recognized due to underreporting and inadequate or no surveillance.8, 9
In an overview of infections associated with flexible bronchoscopy from 2013, 50 studies were identified. In 30 out of the 50 studies published the same contaminant was found in the patient as well as in the bronchoscope. A total of 569 contaminated patients and 115 infected patients (20.21%) could be directly related to contaminated bronchoscopes.8
Routine cleaning does not effectively remove biofilm from endoscope channels. Biofilm was present in 13 out of 13 endoscopes despite appropriate cleaning procedures being followed in the channels of 12 out of 13 instruments.10
Accordingly another study found, microbial growth in 32 of 45 endoscopes (71%).17
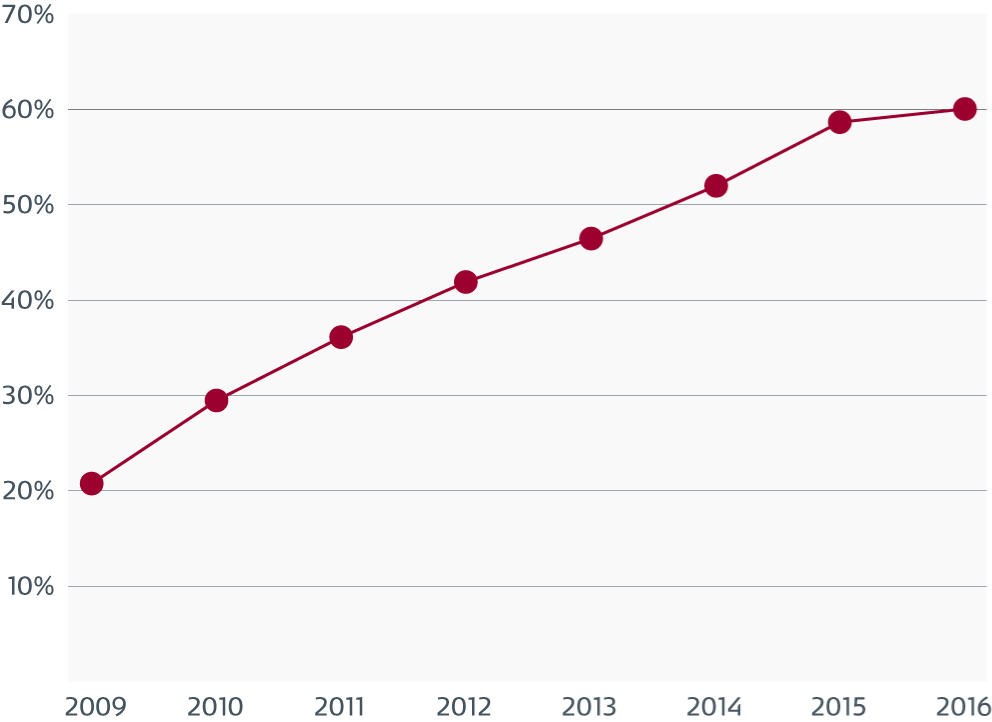
Soil and micro-organisms in suction channels
Electron micrographs of a suction channel that show surface defects.
The biological soil is associated with the defects and is also attached to undamaged areas.

A magnification of one of the defect areas that shows soil and various types of micro-organisms.
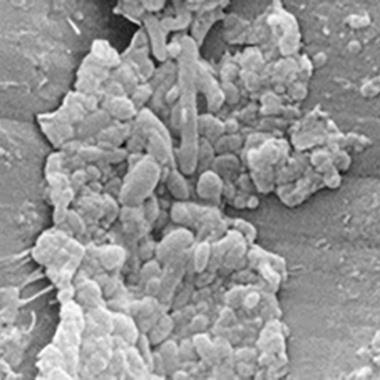
Soil and biofilm in air/water channels
Electron micrographs of two different air/water channels with biofilms.
A low-power view showing a confluent layer of soil and biofilm.
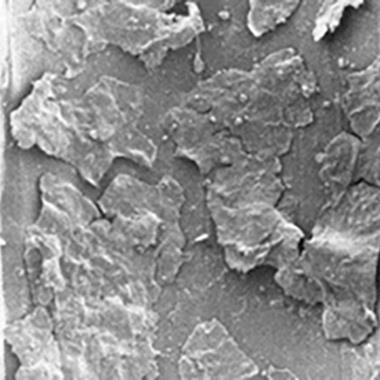
A multilayered biofilm consisting of healthy-looking cells surrounded and overlayed with amorphous-looking exopolysaccharides.
The risk of resistant bacteria strains is increasing
Intensivists, hygiene nurses and others involved in infection control have been aware of the risk of contamination and infection of patients under medical care for many years. The arrival of Multi-Drug Resistant Organisms (MDRO) such as CRE or multidrug resistant Pseudomonas aeruginosa constitutes a new challenge when it come to the risks involved for patients, physicians, hospitals and clinics.
Outbreaks of drug resistant organisms caused by reusable bronchoscopes have been reported.18,19,20
Watch the video that demonstrates how bronchoscopes pose a particular risk of patient to patient cross-contamination.
Financial impact of cross-contamination
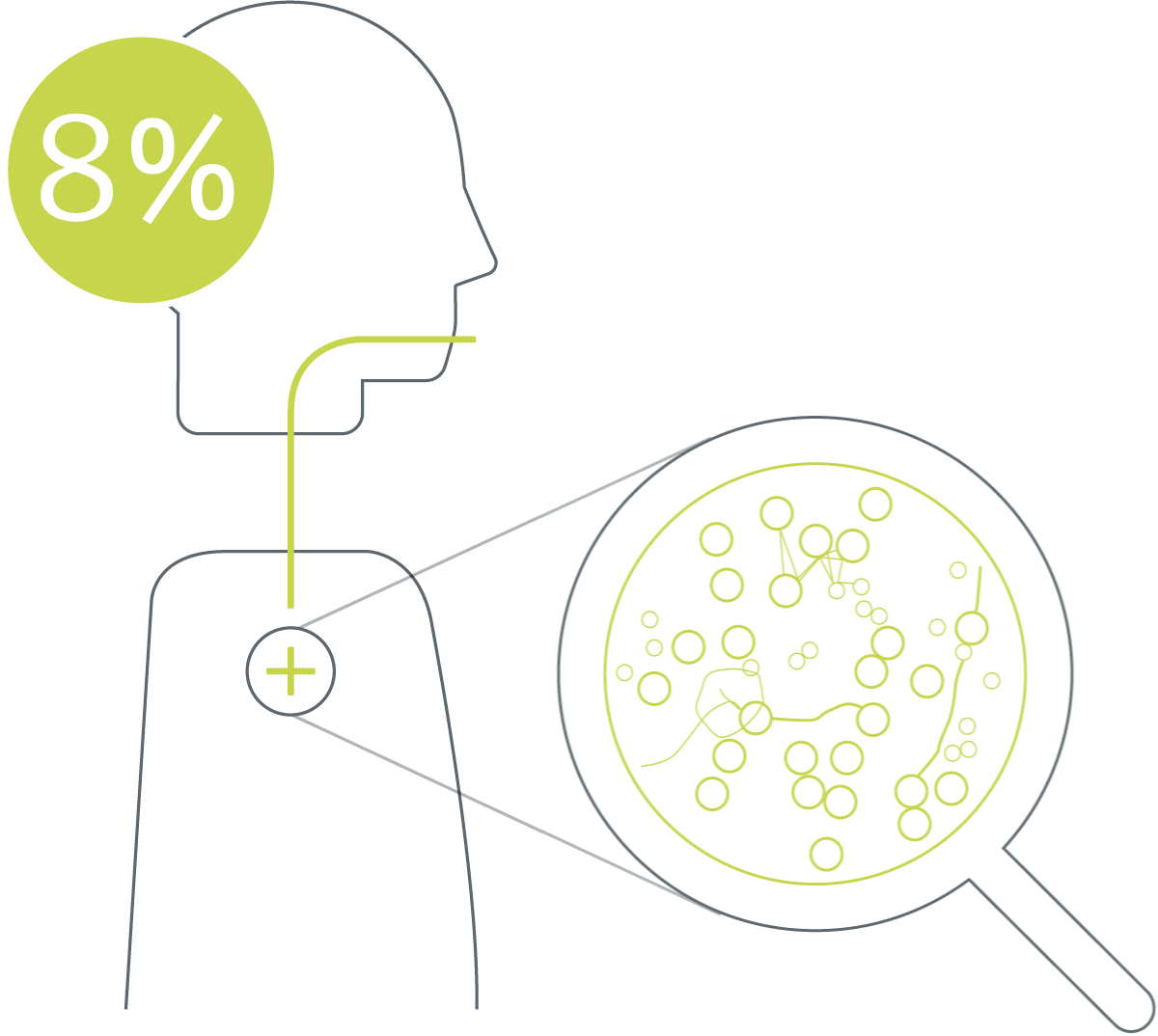
Quantifying the results of a systematic literature search revealed an overall contamination of bronchoscopes of 8.7%.* The analysis includes 1664 samples from 13 studies conduced in 8 different countries.25-37
As the majority of patients contaminated from bronchoscopes suffer from pneumonia8,13 costs linked to ventilator associated pneumonia ($25.149) are used as the clinical impact in the calculation of costs. Combining the 8% risk of cross-contamination with the 20,21% infection risk, the cost associated with cross-contamination can be calculated:
0.08 x 0.2021 x 25.149 = $407*
*Depending sources, risk of contamination and infections plus cost of VAP.
8% risk of a cross-contamination25-37 times the risk of an infection (calculation based on Kovaleva et al.8) times the cost of a ventilator-associated pneumonia $25.149.38
In a recent Pseudomonas outbreak, the health-care costs directly related to the diagnosis, treatment, and hospitalization of the six affected patients were estimated to be $243.000 or $40.500 per patient.14
Single use. New possibilities.
Outbreaks have led physicians to question the safety of bronchoscopy. Endoscopes, including bronchoscopes, are the medical devices most frequently associated with outbreaks of nosocomial infections.15, 16
The risk of cross-infection with multi-resistant microbes in the ICU during bedside bronchoscopy procedures can be significantly reduced by using a sterile single-use bronchoscope.
Ambu’s single-use aScope 4 Broncho minimizes the risk of cross-contamination by ensuring sterility straight from the pack, thus avoiding residual biofilm caused by inadequate automatic endoscope reprocessing.
Want to know more?
Do you want to now more? Fill in your contact information in the form and we will contact you.
References
CDC Guideline 2008. Disinfection and Sterilization in Healthcare Facilities.
US senate report January 13, 2016 Preventable Tragedies: Superbugs and How Ineffective Monitoring of Medical Device Safety Fails Patients
ECRI Institute https://www.ecri.org/Pages/default.aspx
FDA News Release. FDA releases final guidance on reprocessing of reusable medical devices. March 12, 2015.
Reprocessing Medical Devices in Health Care Settings: Validation Methods and Labeling. Guidance for Industry and Food and Drug Administration Staff. FDA. March 17, 2015.
Go to article: Infections Associated with Reprocessed Flexible Bronchoscopes: FDA Safety Communication
Go to: FDA MAUDE database
Kovaleva et al. 2013; Transmission of Infection by Flexible Gastrointestinal Endoscopy and Bronchoscopy. Clinical Microbiology Rev. April 2013 vol. 26 no. 2, p. 231-254
MM. Mughal et al.2004; Reprocessing the Bronchoscope: The Challenges, Seminars in Respiratory and Critical Care Medicine. Vol. 25 no. 4, p. 443-449
Pajkos et al. Is biofilm accumulation on endoscope tubing a contributor to the failure of cleaning and decontamination? Journal of Hospital Infection 2004, 58:224-229.
Terjesen et al. 2017; Early Assessment of the Likely Cost Effectiveness of Single-Use Flexible Video Bronchoscopes
Michelle Alfa Ph.D.; Endoscope Reprocessing Verification Testing, Presentation, Meeting Materials Non-FDA Generated, FDA Committee Meeting, May 14-15, 2015
R. Douglas Scott II, The Direct Medical Costs of Healthcare-Associated Infections in U.S. Hospitals and the Benefits of Prevention, Division of Healthcare Quality Promotion, National Center for Preparedness, Detection, and Control of Infectious Diseases, Centers for Disease Control and Prevention, March 2009
Kovaleva et al. Usefulness of Bacteriological Monitoring of Endoscope Reprocessing, Therapeutic Gastrointestinal Endoscopy, Chap. 9, p. 141-162, 2011
Srinivasan et al. An Outbreak of Pseudomonas aeruginosa Infections Associated with Flexible Bronchoscopes. The New England Journal of Medicine, 2003; 348;221-7
CDC release from the Healthcare Infection Control Practices Advisory Committee (HICPAC) January 25, 2017. "Essential Elements of a Reprocessing Program for Flexible Endoscopes – Recommendations of the HICPAC”
Cori L. Ofstead et al 2018; Residual moisture andwaterborne pathogens inside flexible endoscopes: Evidence from a multisite study of endoscope drying effectiveness
T. Waite et al 2016; Pseudo-outbreaks of Stenotrophomonas maltophilia on an intensive care unit in England.
Janine Zweigner et al 2014; A carbapenem-resistant Klebsiella pneumoniae outbreak following bronchoscopy
T Agerton et al 1997; Transmission of a highly drug-resistant strain (strain W1) of Mycobacterium tuberculosis. Community outbreak and nosoco- mial transmission via a contaminated bronchoscope.
Ofstead et al 2017: Longitudinal assessment of reprocessing effectiveness for colonoscopes and gastroscopes: Results of visual inspections, biochemical markers, and microbial cultures
Kovaleva and Buss: Usefulness of Bacteriological Monitoring of Endoscope Reprocessing
The Joint Commission: Quick Safety Issue 33 May 2017
Ofstead et al. 2018 “Effectiveness of reprocessing for flexible bronchoscopes and endobronchial ultrasound bronchoscopes” CHEST
M. Guy et al. “Outbreak of pulmonary Pseudomonas aeruginosa and Stenotrophomonas maltophilia infections related to contaminated bronchoscope suction valves” Europe's journal of infectious diseases epidemiology, prevention and control Eurosurveillance, Volume 21, Issue 28, 14 July 2016
C. Ofstead et al 2016 “Practical toolkit for monitoring endoscope reprocessing effectiveness: Identification of viable bacteria on gastroscopes, colonoscopes, and bronchoscopes.”
Cori L. Ofstead et al. 2018 “Residual moisture and waterborne pathogens inside flexible endoscopes: Evidence from a multisite study of endoscope drying effectiveness.”
P. Batailler et al 2015 “Usefulness of Adenosinetriphosphate Bioluminescence Assay (ATPmetry) for Monitoring the Reprocessing of Endoscopes.”
M. Botana-Rial et al 2016 “A Pseudo-Outbreak of Pseudomonas putida and Stenotrophomonas maltophilia in a Bronchoscopy Unit.”
C. DiazGranados et al 2009 “Outbreak of Pseudomonas aeruginosa Infection Associated With Contamination of a Flexible Bronchoscope”
L. Gavaldà et al 2015 “Microbiological monitoring of flexible bronchoscopes after high-level disinfection and flushing channels with alcohol: Results and costs”
T. Guimarães et al 2016 “Pseudooutbreak of rapidly growing mycobacteria due to Mycobacterium abscessus subsp bolletii in a digestive and respiratory endoscopy unit caused by the same clone as that of a countrywide outbreak”
M. Marino et al 2012 “Is Reprocessing After Disuse a Safety Procedure for Bronchoscopy?”
D. Rosengarten et al 2010 “Cluster of Pseudoinfections with Burkholderia cepacia Associated with a Contaminated Washer ‐ Disinfector in a Bronchoscopy Unit”
N. Shimono et al 2008 “An outbreak of Pseudomonas aeruginosa infections following thoracic surgeries occurring via the contamination of bronchoscopes and an automatic endoscope reprocessor.”
S. Vincenti et al 2014 “Non-fermentative gram-negative bacteria in hospital tap water and water used for haemodialysis and bronchoscope flushing: Prevalence and distribution of antibiotic resistant strains”
T.D. Waite et al “Pseudo-outbreaks of Stenotrophomonas maltophilia on an intensive care unit in England.”
R. Douglas 2009 “The Direct Medical costs of Healthcare-Associated Infections in U.S. Hospitals and the Benefits of Prevention”

