Reusable medical devices pose a risk of infection through cross-contamination
Hospital-acquired infections (HAIs) are among the most frequent adverse events occurring in healthcare. Besides causing considerable discomfort for patients, they also cause significant economic burden on healthcare systems.1,2
On any given day, about one in 25 hospitalized patients has at least one healthcare-associated infection.1
Did you know ...
Electroencephalography (EEG) cup electrodes are available in both reusable and single-use versions. The single-use version makes it possible to minimize the risk of cross-contamination.3
High-level disinfection
Before applying EEG cup electrodes to the scalp, the skin is cleaned and abraded to remove the epidermal layer of the skin.4,5 Because of the abrasion EEG cup electrodes are categorized as semi-critical devices. They require high-level disinfection (HLD) or sterilization.6,7
If EEG cup electrodes become contaminated because of inadequate cleaning and are used on a patient, there is a risk of infection.
Total cost of reusable EEG cup electrodes
To identify the total cost of using reusable EEG cup electrodes, both Cost of Use and Cost of Clinical Outcome must be determined.
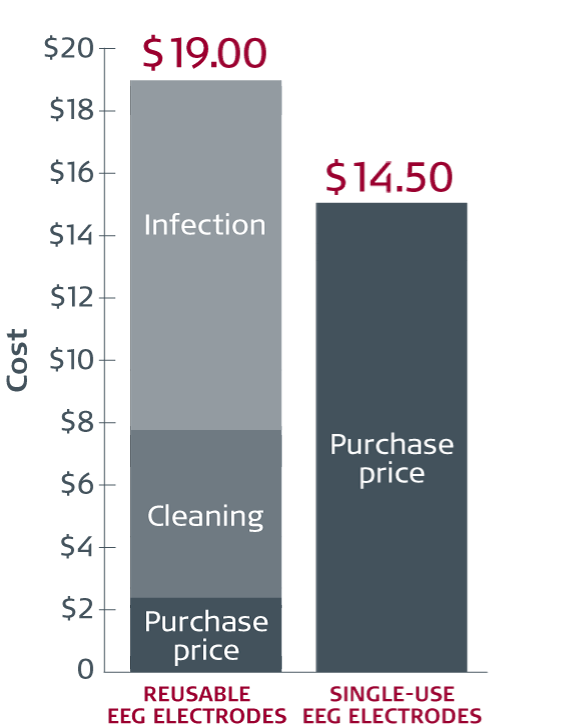
Reusable vs single-use cup electrodes
Average cost per procedure12
The rate of contamination and risk of sepsis
A 2018 study investigating pathogen growth and the risk of contamination on cleaned, ready-to-use EEG cup electrodes. The study revealed that 1 out of 4 EEG cup electrodes harboured bacteria. 88% (7 of 8) of the bacteria identified were categorized as potential or at-risk of infections.8
Did you know ...
Sepsis is a severe syndrome that can lead to tissue damage, organ failure, and death, and it is very expensive to treat. 11% of all sepsis cases start with a skin infection.9,10
Cost of Clinical Outcome (risk of infection)
To identify the risk of sepsis, a systematic study was designed using data from Truven Health Analytics, with a total of 73,834 patients.11 The patients investigated had two outpatient visits; at the first visit, the patient did not have an EEG procedure; at the second visit, the patient received an EEG procedure. The incidences of sepsis were identified 14 days after the two visits, respectively.
- 0 patients had sepsis 14 days after the non-EEG visit
- 24 patients had sepsis 14 days after the EEG procedure
(33 cases per 100,000 EEG procedures – 0.033 %)
The cost of sepsis was identified via a review assessing the hospital-related cost of sepsis. More than 9 million patients were included. The mean hospital-wide cost of sepsis per patient was USD 33,718.9
Cost of use
Based on studies from 4 US hospitals, where all 4 were using HLD as the cleaning method, the combination of acquisition and cleaning costs equates to an average of approximately USD 9 per use. Costs vary from hospital to hospital. At one hospital, the combination of acquisition and cleaning costs was more than USD 15 per use.
Collected data included:
- Costs related to acquisition
- Time spent on cleaning
- Cleaning of the electrodes
The acquisition cost per procedure was estimated at USD 18.50 for single-use EEG cup electrodes (Ambu® Neuroline Cup).
Test single-use EEG electrodes for free
The Ambu Neuroline Cup electrode is for single patient use, specially designed for clinical EEG, EP and PSG examinations. The consistent and superior signal quality as well as greater convenience.
The use of single-use electrodes eliminates the risk of cross-contamination.
Get a free sample
Click the button and order a free sample of the Ambu Neuroline Cup Electrode to test it out on your facility.
Contact me, I want to learn more!
To learn more about the interaction between cross-contamination, the use of reusable EEG electrodes, the cost and the complexity of cleaning, please sign up for a noncommital meeting with your Ambu representative.
References
World Health Organization. Report on the Burden of Endemic Health Care-Associated Infection Worldwide. WHO Libr Cat Data; 2011.
Burke JP. Infection Control — a problem for patient safety. N Engl J Med. 2003;348:651–6.
Mazzaro N. Ambu Disposable Cup Electrode. 2010. Accessed 6 Dec 2017.
Teplan M. Fundamentals of EEG measurement. Meas Sci Rev 2002;2:1–11.
Ferree TC, Luu PL, Russel GS, Tucker DM. Scalp electrode impedance, infection risk, and EEG data quality. J Chem Inf Model 2013;53:1689–99.
Rutala WA, Weber DJ, The Healthcare Infection Control Practices Advisory Committee (HICPAC). Guideline for Disinfection and Sterilization in Healthcare Facilities, 2008. Centers for Disease Control and Prevention; 2008. Accessed 6 Dec 2017.
Scott NK. Infection prevention: 2013 review and update for neurodiagnostic technologists. Neurodiagn J 2013;53:271–88.
Albert N, Bena J, Runner J, Morrision J, Ciudad, Rice K, Keleekai N, Slifack. Contamination of Reusable EEG Electrodes, A Multicenter Study. 2018 poster presentation at APIC. Session 2103.
Arefian H, Heublein S, Martin F, Younis MZ, Moerer O, Fischer D, et al. Hospital-related cost of sepsis: a systematic review. J Infect 2017;74:107–17.
Centers for Disease Control and Prevention. Sepsis. 2017. Accessed 6 Dec 2017.
IBM Watson Health www.truvenhealth.com
Sohrt A, et. al. PharmacoEconomics - Open 2018, Cost-effectiveness Analysis of Single-Use EEG Cup Electrodes Compared with Reusable EEG Cup Electrodes


