Reusable EEG electrodes could be a source bacteria causing HAI1
Did you know that EEG electrodes are classified as semi-critical devices?2 That means they are subject to high-level disinfection requirements3 – the same as bronchoscopes and colonoscopes.
Insufficient cleaning of reusable EEG electrodes can lead to hospital-acquired infections (HAI).1 A report by WHO says that HAI are the most frequent adverse events in healthcare. 7-10% of all hospitalized patients worldwide will be subject to at least one HAI.3 Treating HAI are costly for the healthcare sector and can be a major burden to patients.1
1 out of 4 reusable EEG
electrodes are contaminated1
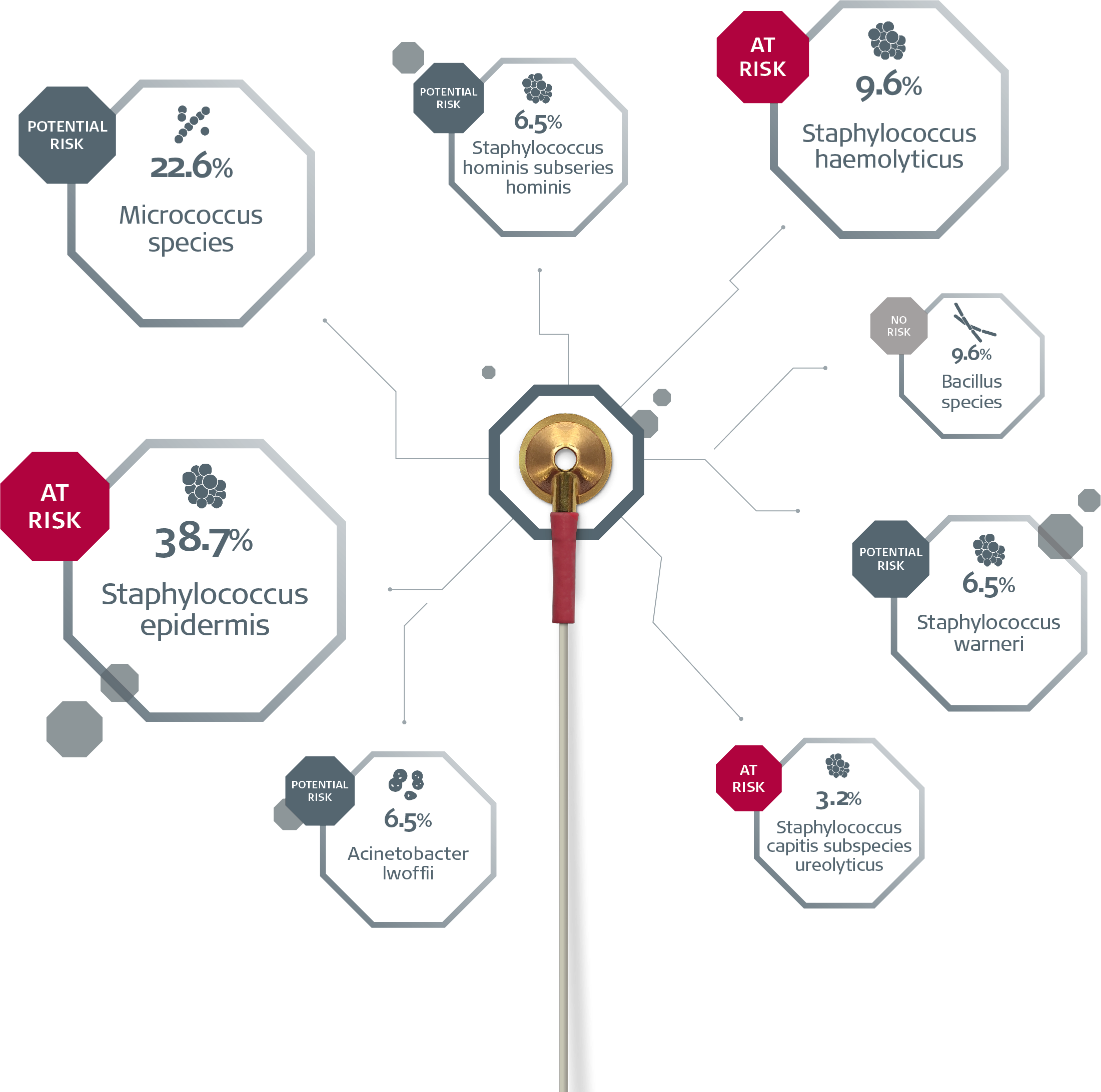
A recent multi-center study examined whether cleaned, ready-for-patient-use reusable EEG electrodes harboured bacteria that could lead to human infection.1
1 out of 4 were found to be contaminated.1 The positive cultures were labelled by risk of HAIs. 90% of the contaminated, cleaned, ready-to-use reusable EEG electrodes harboured bacteria with the potential to lead to HAI.1
Download summary
Download the summary of the multi-center Study now.

EEG Cup Electrodes - Stop Cross-Contamination
1 out of 4 reusable EEG cup electrodes are contaminated with bacteria. They are complex and costly to clean. Risk bacteria could led to hospital-acquired infection. Stop cross-contamination and use single-use EEG cup electrodes.
Are you putting your patients at risk?
All in- and out-patients are at risk of HAI. Certain patient groups are at a higher risk, such as patients cared for in specialized areas or in high-dependency units.4
EEG electrodes are semi-critical devices applied on abraded skin. Study results show a high number of cleaned ready-to-use reusable EEG electrodes contaminated with at-risk or potential risk bacteria.1 Thus, it may be advisable to re-evaluate cleaning methods and general standard practices for EEG electrodes.1
Alternatively, single-use EEG electrodes may be considered to minimize the potential risk of patient-to-patient cross-contamination.1
See our cup electrode Get free sample
It is costly, complex and time consuming to disinfect
Cleaning alone does not remove bacteria. Disinfection is required to remove microscopic debris, including blood.
There are three levels of disinfection and reusable EEG electrodes require the highest level.3 The cleaning guidelines for this are complex. If followed, the cleaning process takes approximately 20 minutes. Alternatively, guidelines suggest that reusable EEG electrodes could be sterilized by autoclaving.
The cost of disinfection
When following official guidelines, cleaning reusable EEG electrodes is complex and time-consuming. When you combine the cost of reusable EEG electrodes with the cost (and time) spent on cleaning them, reusable EEG electrodes can become expensive devices.
Your hospital can reduce the risk of HAI
Many hospitals have adopted strict disinfection techniques as part of a multi-barrier strategy to prevent HAI. To eliminate the potential risk of cross-contamination, single-use EEG electrodes could be considered.1
Test single-use EEG electrodes for free
The Ambu Neuroline Cup electrode is for single patient use, specially designed for clinical EEG, EP and PSG examinations. The consistent and superior signal quality as well as greater convenience.
The use of single-use electrodes eliminates the risk of cross-contamination.
Get a free sample
Click the button and order a free sample of the Ambu Neuroline Cup Electrode to test it out on your facility.
Contact me, I want to learn more!
To learn more about the interaction between cross-contamination, the use of reusable EEG electrodes, the cost and the complexity of cleaning, please sign up for a noncommital meeting with your Ambu representative.
References
N. M. M. Albert et al (2018) Contamination of Reusable Electroencephalography Electrodes: A Multi-Center Study
Occupational Safety and Health Administration (OSHA) requirements
N. K. Scott (2013) Infection Prevention: 2013 Review and Update for Neurodiagnostic Technologists, The Neurodiagnostic Journal, 53:4, 271-288
International Organisation of Societies for Electrophysiological Technology (OSET) – Guidelines for infection control in clinical Neurophysiology department

